
Farhang Hooshmand1, Amir Hossein Hassani2, Amir Reza Bahadori2, Zahra Hooshanginezhad3 and Mohammad Shojaie4*
1Assistant Professor of Pathology, Department of Pathology, School of Medicine, Jahrom University of Medical Sciences (JUMS), Jahrom, Iran.
2School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
3Clinical Cardiologist, Jahrom University of Medical Sciences. Jahrom, Fars, Iran.
4Professor of Cardiology. Interventional Cardiologist, Cardiology Department, Jahrom University of Medical Sciences. Jahrom, Fars, Iran.
*Corresponding Author: Mohammad Shojaie, Professor of Cardiology. Interventional Cardiologist, Cardiology Department, Jahrom University of Medical Sciences. Jahrom, Fars, Iran.
Received Date: March 19, 2024
Accepted Date: March 30, 2024
Published Date: April 05, 2024
Citation: Farhang Hooshmand, Amir Hossein Hassani, Amir Reza Bahadori, Zahra Hooshanginezhad and Mohammad Shojaie. (2024) “Esculetin Modulation of Lipid Profiles in White New Zealand Rabbits: A Case-Control Study.”, International Journal of Medical Case Reports and Medical Research, 2(4); DOI: 10.61148/2994-6905/IJMCRMR/035.
Copyright: © 2024. Mohammad Shojaie. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Dyslipidemia is a major condition that closely correlates with cardiovascular disease. Several kinds of medication, such as statins, fibrates, and so on, could control this condition. However, all of these medications have several adverse effects. Esculetin is a natural derivation produced from coumarin. It could be helpful as a new drug in the treatment of dyslipidemia as it has promising anti-inflammatory, anti-diabetic, anti-adipogenic, and antineoplastic effects.
Methods: We evaluated the impact of esculetin among 28 rabbits that got a high-fat diet. The rabbits were weighted and categorized randomly into three groups. Then lipid profile was checked and high-fat diet was started. The third group also got esculetin beside the diet. After sixty days (on day sixty-one), lipid profiles were checked from our subjects. The data were evaluated with IBM SPSS version 26.0.
Result: the second and the third group showed a significant increase in total cholesterol levels after intervention induced (P=0.001 and 0.007, respectively). However, although the second and third groups did not differ prior to the intervention, the total cholesterol level means of the third group was significantly lower than the second group after the treatment with esculetin (696 mg/dL and 290 mg/dL, respectively) (P=0.08 and 0.022, respectively).
Conclusions: Our study illustrates that the subjects taking esculetin have lower total cholesterol and triglyceride levels. Moreover, it could prevent weight gain in the rabbits received high-fat diet.
Introduction:
Background:
Dyslipidemia is an important condition closely related to major cardiovascular events and stroke. Cardiovascular diseases account for the highest proportion of mortality and morbidity worldwide; consequently, better control of its risk factors, such as dyslipidemia, can significantly enhance global public health. During recent decades the global burden of dyslipidemia slightly increased, and disability-adjusted life years (DALYs) of high low-density lipoprotein (LDL) Cholesterol plasma levels have mounted in recent years [1]. The American Heart Association (AHA) describes dyslipidemia as an increase in fasting lipid profile, which contains a total cholesterol level equal to or above 240 mg/dl, LDL cholesterol more than 160 mg/dl, and triglyceride (TG) above 200 mg/dl, or high-density lipoprotein (HDL) cholesterol level less than 40 mg/dl. Due to the importance of dyslipidemia in cardiac events calculating the 10-year risk for stroke and heart diseases is mandatory [2-4].
The goal of treatment in dyslipidemia is to reduce cardiovascular events and normalize LDL cholesterol levels. In addition, triglyceride and HDL cholesterol levels are other treatment priorities. Various drugs are prescribed in dyslipidemia treatment, for instance, statins, fibrate, bile acid sequestrants, ezetimibe, niacin, and omega-3. Statins, such as atorvastatin, rosuvastatin, and fluvastatin which inhibit the hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, are used solely in most cases as the initial treatment to reduce blood LDL cholesterol and total cholesterol levels while others are usually prescribed as combination therapy. Previously several complications reported in using the drugs existed for the treatment of dyslipidemia. Myositis and abnormal liver function tests are the most serious adverse effects of statins. Gemfibrozil and fenofibrate are categorized in the fibrate group, which has an extreme effect on very-low-density lipoprotein (VLDL) cholesterol levels and are widely used in patients with a high level of triglyceride. The most prevalent complication of fibrates are renal and gallbladder diseases, skin rash, and nausea. Bile acid sequestrants are almost safe drugs since they have no absorption through the gastrointestinal tract and reduce cholesterol levels by inhibiting the reabsorption of bile acids in the ileum; however, because of their unpleasant taste and gastro-intestinal side effects, most of the patients discontinue their medications. Ezetiamab and niacin are other medications that could cure dyslipidemia, although liver problems are possible to happen [3-5] (Figure. 1)
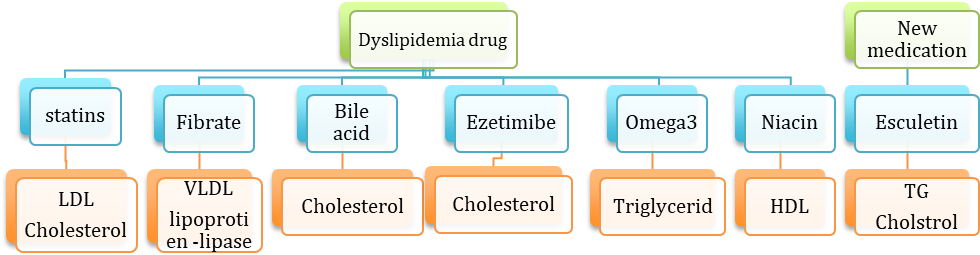
Figure 1: Effect of different drugs on lipid profile
Considering these wide ranges of adverse effects, finding a drug with higher efficacy and lower side effects, is necessary. Esculetin is a natural derivative of coumarin with a chemical formula of C9H6O4, which can be extracted from various herbal plants such as the Fraxinus genus, Citrus limon, and Osbeck [6]. When the hydroxyl group was added to the 6th and 7th carbon molecule of coumarin, esculetin is made. It has effects on several cell-molecular pathways, for instance, gene-regulating processes, protein expressing, and cell signaling pathways [6-8]. Several studies have shown that esculetin has analgesic [9], anti-inflammatory [10], anti-diabetic [10], anti-adipogenic [11], antineoplastic [12], and anti-oxidant effects [6, 13-15].(Figure. 2). Also, this substance inhibits various enzymes such as Cyclooxygenase [6], lipoxygenase-5 [17,18], Catechol-O-methyltransferase [19], and NADPH oxidase [20].
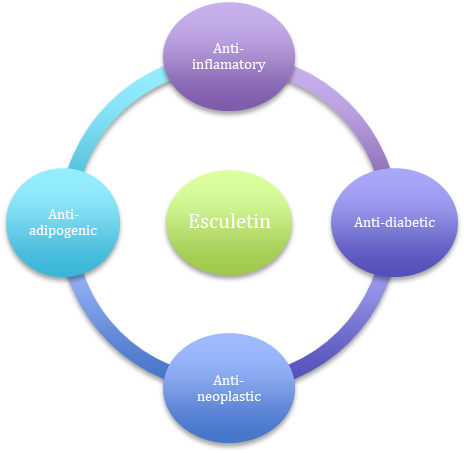
Figure 2: Different mechanisms that esculetin could induce in natural cells.
Lipoxygenase catalyzes molecular oxygen in unsaturated fatty acids in the form of 1-4-cis result in oxidation of lipoproteins which leads to atherosclerosis [21,22]. Esculetin inhibited the lipoxygenase process. Thus, it could help to prevent atherosclerosis. Zhang, L. et al. illustrated that this natural dihydroxy coumarin could reduce LDL cholesterol levels and increase HDL cholesterol thus, the rate of atherosclerosis and hyperlipidemia would diminish. Esculetin, by LDL choleterol oxidation and inhibiting matrix metalloproteinase-6 (MMP-6), has been shown promising effects in treating atherosclerosis [6]. Pullaiah, C. P. et al., in their study on mammalian subjects, demonstrated that esculetin is able to exert cardio-protective effects in myocardial infarction [15]. However, evidence regarding its prophylactic effect on cardiovascular diseases is lacking. From this issue, our study is novel. In the present study, due to lack of trials on the protective impact of esculetin, we aimed to assess the efficacy of esculetin in treatment of dyslipidemia, a major risk factor of cardiovascular events, in New Zealand rabbits so that further studies with human subjects would be conducted to better address this issue.
Material and Methods:
This interventional study followed an animal clinical trial protocol, and all the procedures were reviewed and approved by the Committee for Animal Experiments at the Jahrom University of Medical Sciences. The trial was performed on thirty male white New Zealand rabbits weighing approximately between 1.8 kilograms (kg) and 2.3 kg and was bought from the Pasteur institution in Tehran, Iran. The rabbits were housed individually under the controlled condition at a temperature of 22±2°centigrade, constant humidity, and light and dark cycles of twelve hours.
Also, the rabbits had been fed a regular standard diet for sixty days. Besides, rabbits with the relatively same amount of weight are kept together. The subjects were randomly divided into three various groups; however, unfortunately, two subjects deceased before the initiation of the experiments. Thus, twenty-eight rabbits entered the study; eight rabbits were allocated to the control group (Grope 1), ten subjects to a “high-cholesterol diet without esculetin” group (Grope 2), and the rest of them to the “high cholesterol diet plus esculetin” group (Grope 3). Also, the rabbits were weighed before and after the intervention. Previous to the intervention implementation, rabbits’ ears were shaved with razor baldness and numbed with Xylocaine 10mg spray. Then blood samples were taken from rabbits’ ear arteries with an insulin needle. We chose rabbits' ear vessels owing to many studies recommending that these vessels are extremely easy to access and that they could not harm rabbits [3]. After that, the fasting lipid profile was sent to the laboratory for the measurement of TG, total Cholesterol, LDL cholesterol, and HDL cholesterol.
Then, the experimental diet started for the rabbits. The initial group of eight rabbits was fed via a syringe with 3cc/day of olive oil; the second group of ten rabbits was given 3cc/day of olive oil and 0.5 g/Kg/day of cholesterol, while the third group was fed with 3cc/day of olive oil, 0.5 g/Kg/day of cholesterol, and 10 mg/Kg of our experimental drug, esculetin. Finally, on day sixty-one, the rabbits were weighed again. A blood sample was taken from the subject's other side ear and referred to the same laboratory for measurement evaluation. We should mention that 12 hours of fasting were achieved prior to the last samplings from the subjects.
A photometric method using Biotik, Iran kits had used for the measurement of TG, total Cholesterol, HDL cholesterol, and LDL cholesterol serum levels. The data was entered into IBM SPSS version 26.0. the difference between the mean weights of the groups was evaluated using a one-way Analysis of Variances (one-way ANOVA) test and Tukey post hoc test. Paired T-test was used to assess the efficacy of esculetin in the case group.
Results:
The subjects’ pre-intervention weight in all three groups did not have any significant difference, which addresses that the subjects disturb in all groups equally (P=0.9). In contrast, the weight of the second group, which got high cholesterol diet prominently, increased, and it is obvious due to the diet (P= 0.02). Interestingly, the third group, which dieted with our experimental drug esculetin did not show a significant increase in weight which means that esculetin did not lead to weight gain and also it might have a preventable effect (P=0.71). Furthermore, the comparison between groups 2 and 3 showed that our intervention has a meaningful effect on preventing weight gain in the group which takes esculetin (p=0.01).
Additionally, the second and the third group showed a significant increase in total cholesterol levels after intervention induced (P=0.001 and 0.007, respectively) due to their high-cholesterol diet. However, although the second and third groups did not differ prior to the intervention, the total cholesterol level means of the third group was significantly lower than the second group after the treatment with esculetin (696 mg/dL and 290 mg/dL, respectively) (P=0.08 and 0.022, respectively).
The post-intervention triglyceride level of the second group depicts significant increases with a mean of 110.6 mg/dL (P=0.032), while the third group showed a non-significant decrease in their triglyceride level with a mean of 53.1 mg/dL (P=0.236). In other words, in a group, which got esculetin, the difference between pre and post-intervention means and variances has a prominent deviation (P=0.05).
In addition, although in both intervention groups, the HDL cholesterol levels mounted (P=0.003 and 0.008, respectively), the increases in HDL cholesterol levels were more prominent in the second group. It might relate to the significant differences regarding the higher HDL cholesterol levels of the second group prior to the intervention(P=0.012). The HDL level means of the second group before intervention was 17.1 mg/dL; however, for the third group was 12.4 mg/dL.
The outcomes of esculetin therapy on LDL cholesterol levels were more favorable since both intervention groups illustrated a prominent surge in their LDL cholesterol levels (P=0.002 and 0.002, respectively). However, after the intervention, the third group had lower LDL choleterool levels, but the difference was not statistically significant (P=0.132).
It should mention that the pre-interventional measurement of weight, total cholesterol, HDL cholesterol, and LDL cholesterol levels among all three groups have no significant differences (P= 0.90, 0.08, 0.07, and 0.93, respectively). It depicted that the disturbance of the rabbits is the same in all three groups, and our post-intervention data are not related to chance. Table 1 summarizes the results of pre-intervention and post-intervention measurements of the weight and lipid profile of the subjects.
|
Variable |
Group 1 |
Group 2 |
Group 3 |
p-value (groups 2 and 3) |
|
|
Weight |
Before |
2034±163 |
2115±194 |
2105±172 |
0.904 |
|
After |
2333±166 |
2419±287 |
2136±187 |
0.018 |
|
|
P-value (paired T test) |
0.001 |
0.024 |
0.719 |
|
|
|
Total Cholesterol |
Before |
32.38±9.5 |
35.4±12.2 |
31.4±8.55 |
0.08 |
|
After |
25.37±7.85 |
696±457.74 |
290.1±230±43 |
0.022 |
|
|
P-value (paired T test) |
0.159 |
0.001 |
0.007 |
|
|
|
triglyceride |
Before |
54.38±14.74 |
45.2±11 |
67.8±11.22 |
<0.001 |
|
After |
37±5.53 |
110.6±79.76 |
53.1±33.37 |
0.05 |
|
|
P-value (paired T test) |
0.009 |
0.032 |
0.263 |
|
|
|
HDL |
Before |
88.31±14.3 |
17.1±7.47 |
12.4±1.01 |
0.079 |
|
|
After |
82.6±13.38 |
55.4±30.5 |
26±13.2 |
0.012 |
|
P-value (paired T test) |
0.595 |
0.003 |
0.008 |
|
|
|
LDL |
Before |
14.75±4.98 |
12.5±2.32 |
12.6±2.79 |
0.939 |
|
After |
8.38±3.38 |
227.159±2.76 |
136.87±3.93 |
0.132 |
|
|
P-value (paired T test) |
0.036 |
0.002 |
0.002 |
|
|
Table 1: The weight and lipid profile of the subjects in the study
Discussion:
In the present article, we evaluated the effect of esculetin in the enhancement of lipid profile in the murine specimens. The samples were allocated to three groups fed with high-lipid diets, with one of them consuming esculetin as well. We found that esculetin was significantly effective in the reduction of weight, triglyceride, and total cholesterol. It could also decrease the level of LDL cholesterol and HDL cholesterol, but the clinical outcome was not statistically significant.
These days, owing to a sedentary lifestyle, high-fat diet, metabolic syndrome, and stressful lifestyle, many individuals suffer from hyperlipidemia, which could mount the risk of cardiovascular diseases [24]. Today used medications to treat dyslipidemia have several adverse effects. For example, statins induced abnormal liver function test, fibrates may present renal insufficiency, and so on [4]. Hence, a new drug with lower adverse effects is well sensed. Esculetin (6,7-dihydroxycumarin) is a kind of pharmaceutical agent which is produced as a lipoxygenase inhibitor derived from coumarin and is found naturally in many herbal plants such as Cortex fraxini. esculetin is found to have antineoplastic, anti-inflammatory, anticoagulative, antioxidative, antibacterial, and antidiabetic effects. Esculetin is also found to induce apoptosis in adipocytes, and thus, decrease the adipose mass of the body and decrease the risk of metabolic syndrome and cardiovascular diseases [25-27]. Thus, esculetin could be a suitable choice as a new agent in the treatment of dyslipidemia, even though more researches are mandatory.
In the present animal study, the role of esculetin in the redaction of rabbits’ lipid profile was studied according to the molecular pharmaceutical structure of that. Dozens of studies indicated that esculetin might have a significant role in diminishing TG, LDL cholesterol, and total cholesterol. Notwithstanding, several articles express that it does not play a major role in decreasing lipid profile [25]. Satheesh et al. illustrated that coumarin agents such as esculetin have a dominant role in the redaction of TG, LDL cholesterol, and total cholesterol levels [28]; however, Tasdemir E. et al. showed that there is not a powerful correlation between escultin use and a decrease in lipid profile; also Tesdemir E et al. indicated that esculetin might have a marginal role in alleviating hyperlipidemia symptoms [25]. Therefore, several controversies might exsiccate in this condition. Our results support the theory that esculetin has a prominent role as a dyslipidemia treatment. In addition, figures 3, and 4 illustrate the mean difference of HDL cholesterol, total cholesterol, TG, and LDL cholesterol in our experiment before and after the intervention.
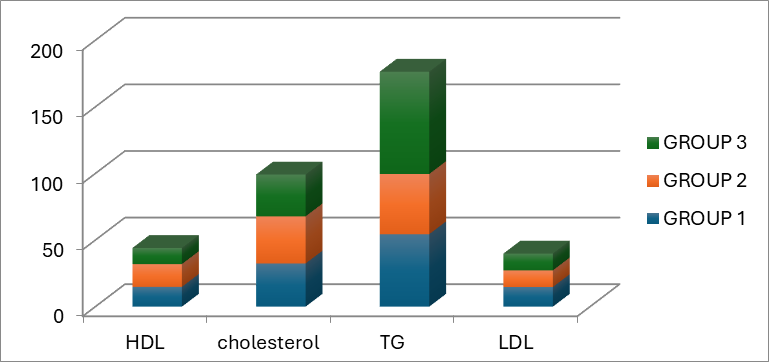
Figure 3: The bar chart depicts the mean of our subject lipid profile before intervention in three separate groups.
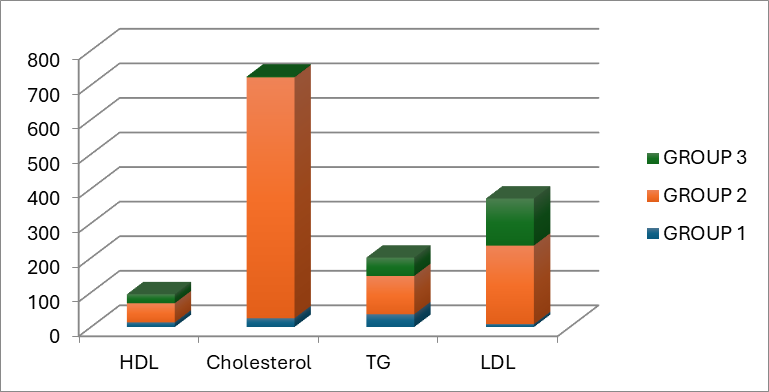
Figure 4: The bar chart gives information about the post-intervention mean of the lipid profile component.
Karthika et al. evaluated the effects of esculetin on lipoperoxides and antioxidants in rats with myocardial infarction induced by Isoproterenol. They found that esculetin would decrease the level of plasma thiobarbituric acid reactive substances (TBARS), plasma hydroperoxides (HP), Heart TBARS, heart HP, and increase the level of Superoxide dismutase, plasma ceruloplasmin, Heart glutathione, as well as Vitamin C and E in the plasma and the heart. However, the levels of the mentioned variables did not differ between control rats and the ones who received esculetin only without Isoproterenol [29]. The findings of Karthika indicated that esculetin could have cardio-protective effects in rats with myocardial infarction but did not have a prophylactic effect. In our study, on the other hand, we found that since esculetin can improve the lipid profile in rabbits, it might have a prophylactic effect against major cardiovascular diseases.
Our animal trial depicts that esculetin significantly reduces the level of TG and Cholesterol; thus, it could help to treat dyslipidemia and diminish the cardiovascular risk of high lipid profile condition. Although numerous studies support our result, Wang et al. illustrated that esculetin is able to reduce the level of LDL cholesterol [24]. In our study, the LDL cholesterol level of the intervention group was reduced, although the result is not significant. Furthermore, numerous studies express that the level of HDL cholesterol was decreased in their studies [24,30,31]; in contrast, Tasdamir E. et al. showed that HDL cholesterol level was increased due to esculetin in their experimental rats. The HDL cholesterol level was mounted in our trial despite it does not have statistically significant meaning. Strongly, further studies with larger sample sizes are necessary to draw a more precise conclusion.
Choi and colleagues also conducted an animal study on diabetic mice with non-alcoholic fatty liver disease, which showed that esculetin could decrease the level of systemic inflammation and reverse the changes seen in the histopathology of the liver during non-alcoholic fatty liver disease [32]. Such findings are in line with our study that esculetin can enhance the condition of patients with dyslipidemia and prevent its negative outcomes.
Conclusions:
In this study, we found that esculetin could prevent weight gain due to high-fat diets; moreover, it was effective in decreasing the total cholesterol and triglyceride levels in New Zealand rabbits. However, it was not able to increase the HDL cholesterol level or decrease the LDL cholesterol level significantly.
Limitations:
It would be better if we could have baseline and post-intervention lab studies such as serum creatinine, liver function tests, and thyroid function tests, among others, to better assess the safety of esculetin in mammalian subjects. Moreover, hepatic and myocardial pathologic specimens would give beneficial information; however, due to the lack of financial resources, it was not possible for us.
Recommendations:
Further studies must be conducted on the safety and efficacy of esculetin in the treatment of dyslipidemia in mammalian subjects so that it could be tested on human subjects later.
Declaration:
Acknowledgments:
Not applicable.
Ethics approval:
This interventional study followed an animal clinical trial protocol and reported in accordance with ARRIVE guidelines. All the procedures were reviewed and approved by the Committee for Animal Experiments at the Jahrom University of Medical Sciences. all experiments were performed in accordance with relevant animal study guidelines and regulations.
Conflict of interests:
The authors declare no conflict of interests.
Consent for publication:
Not applicable
Availability of data and materials:
The datas are also available on request from the corresponding author.
Competing interests:
The authors declare no competing interests.
Funding:
This research received no external funding.